Problem to solve: integrate a solution to get a continuous automated cell counting
Expectations
- Get real time Viable Cell Density (VCD) for making upstream process decisions faster and earlier than with end-point measurements (feeding, infection timeline, etc).
- Ability to continuously collect data, no manual sampling, cell count of importance, reduction of contamination risk, sample consumption.
- Compare with offline counters use trypan blue exclusion method to assess VCD.
- Cell diameter changes, BVES therefore a parameter of interest
- Disposable technology that integrates with Xcellerex XDR-10 or XDR-50 platform and consistent with mostly disposable process
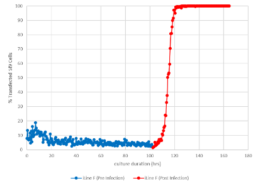
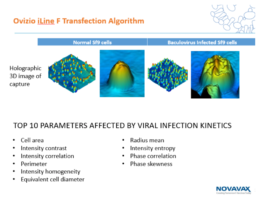
We developed an algorithm to track the infection process of the Sf9 cell lines with the baculovirus. The top 10 cells’ attributes affected by the infection process are listed below.
- Profile of baculovirus infected Sf9 cell culture available on OsOne software
- ~2 – 6 hour lag prior to increase in infected cells
- Based on our data, a successful infection reaches 90 – 100% in 24 – 26 hours
- Multiple reactor runs at 10 and 50 L scale demonstrate similar infection profiles
- These cell culture runs yielded the expected upstream recombinant protein yield
What Novavax says…
Sean Case, Manager of the Upstream Scale Up Group at Novavax
Dear Mr. Case, could you please introduce yourself and your research briefly?
“Sean Case, I’m the manager of the Upstream Scale Up Group at Novavax. At Novavax our group is responsible for defining the process at large scale to transfer to the manufacturing group. We work with Sf9 cells and use a baculoviral expression system to produce the desired recombinant protein. Our group optimizes production bioreactor conditions that are favorable for Sf9 cell growth and protein production while considering factors at the commercial scale.”
What made you aware of Ovizio’s technology?
“While researching technology for continuous cell monitoring in bioreactors I discovered your technology.”
How did you envision the integration of Ovizio’s microscope with your existing process?
“I envisioned Ovizio’s microscope providing us with real-time continuous cell counting that would enable us to make process decisions without the need to sample our reactors. Also, the ability to monitor the morphology of the Sf9 cells and Ovizio’s development of the OsOne software we could capture the real-time status of the baculovirus infection in the production bioreactor.”
Can you comment on the results you have generated and how the technology has helped you improve your process?
“We have generated cell counting results that compare favorably with our off-line counting analytical instrument, and the real-time counting allows us to provide supplementation to our production bioreactor at specific cell densities. The infection algorithm and real-time infection profile allow us to understand if the infection has occurred and how representative it appears to previous production runs.”
According to you, what are the key benefits of the technology? And what were the challenges you have faced during integration?
“The benefits of this technology are the dye-free continuous real-time cell counts, the simple setup and integration with a disposable bioreactor system, the real-time status of the infection kinetics, and using this technology as a PAT approach in developing the upstream process.”
According to your experience, what would be the next step for Ovizio? How could we further improve the technology? What other benefit would you be looking for?
“Increasing the BioConnect cell density capacity to accommodate higher cell densities that come with process improvements. Incorporating the pump closer to the microscope so that critical equipment is located together.”
Thank you very much for your time. It has been great discussing these truly fascinating insights with you!