MSC Profiling in Response to Priming
ASTEM and OVIZIO successfully implemented
a label-free method to monitor human bone marrow MSC morphological profile
Challenge
Mesenchymal stem cells (MSCs) are widely studied as a potential therapy for immune-mediated diseases (osteoarthritis, diabetes, multiple sclerosis, and Alzheimer’s disease). MSCs are known to modulate immune cells such as T cells, B cells, macrophages, microglia, and dendritic cells.
MSCs can be used in an allogeneic, off-the-shelf manner, and have been studied in over 600 clinical trials to date.
However, the manufacture of MSCs with consistent and predictable quality has proven difficult. There is an urgent need to develop imaging systems that are robust and provide real-time monitoring of attributes that are critical to the quality of the stem cells. It is of particular importance that such systems have the ability to not only monitor changes in stem cell phenotype but also their function. Moreover, such a system must also be able to account for donor-to-donor variability.
Solution
Ovizio’s qMod camera captures quantitative phase images from which 30 features related to stem cell phenotype can be extracted (aspect ratio, diameter, elongatedeness, etc). It does so in a label-free manner, easing the burden on quality
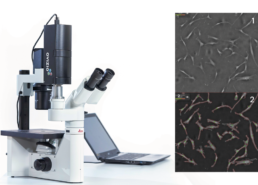
Phase image of MSC culture¹ with mask overlay²
The morphological features can be extracted from phase images and machine learning algorithms can be used to generate phenotypic signatures such as cell count, viability, priming status and immunosuppressive capacity.
Recently, software tools such as t-SNE have been developed to enable easy 2D visualization of complex data, facilitating exploratory research and potential identification of unknown populations.
Experimental Set-Up
We succeeded in establishing the optimal MSC seeding density and priming conditions to capture morphological changes in MSCs isolated from three donors.
We developed a preliminary algorithm to identify multiple MSC morphological features (cell aspect ratio, cell area, elongatedness, optical height) to predict the effects of IFN-γ and TNF-α priming. These observations show a
strong dependence on cell density, irrespective of donor.
Results
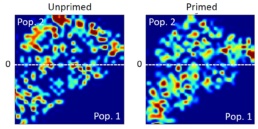
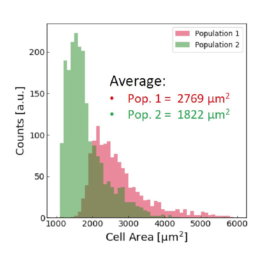
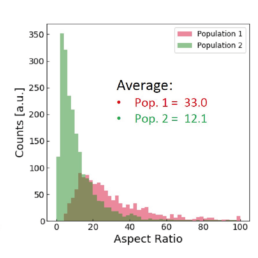
Applying the t-SNE algorithm on the data revealed subpopulation differences for all donors. When primed, there is an increase in MSC subpopulation 1 that corresponds to larger, more elongated, and flatter cells with a higher aspect ratio.
t-SNE algorithm testing on unprimed
and primed MSCs
The distribution of the four cell features in Donor 1 is illustrated above. Even though each donor has the same qualitative differences between Pop. 1 and 2, the feature distribution is donor-dependent.
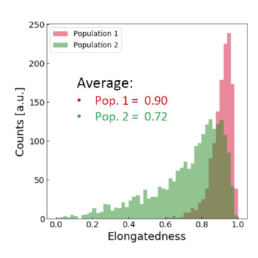
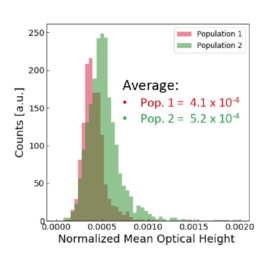
Using all available data, we built a supervised machine learning model (random forest classifier with shallow trees) using the computed cell features as input for the model. The preliminary supervised machine learning model concluded that the effect of priming on MSCs is not dependent on the donor. Rather our study showed:
- an increase in the cell aspect ratio (length/width)
- an increase in the cell area
- an increase in the elongatedness
- a decrease in the optical height
Supervised machine learning model testing on unprimed and primed MSCs
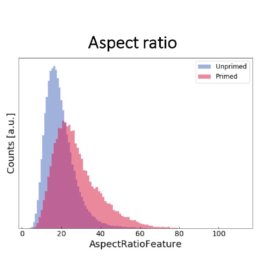
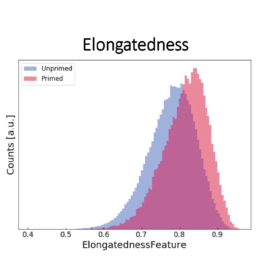
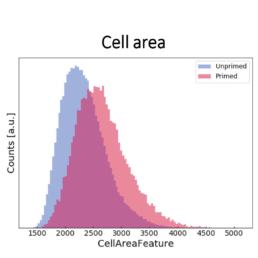
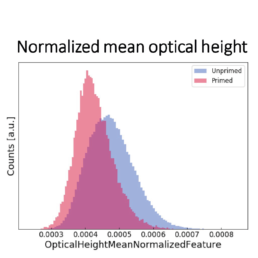
The image-based prediction model showed the distribution of the four cell features in unprimed (blue) and primed MSC populations (pink).
Conclusion
We developed a machine learning algorithm to predict the effect of priming on changes in MSC morphology. We show that primed MSCs are larger, more elongated, and have a flatter and higher aspect ratio.
These results will be further validated in a comprehensive study using MSCs isolated from 12 additional donors. This will include a correlation between MSC function and morphological changes in response to various priming events. Ultimately, this study seeks to position the qMod imaging system for monitoring the quality of MSCs in the manufacturing workflow.

Contact: Pascale Charbonnel, pascale.charbonnel@ovizio.com
Catherine Chong, chongyf@imcb.a-star.edu.sg
Steve Oh, Simon Cool, Ying Zhang, Yin Lu, Tongming Liu from ASTARSG
Damien Cabosart, Pascale Charbonnel, Emilie Viey from Ovizio
Biofactory Competence Centre partners with Ovizio to improve PAT manufacturing
Share this
About the partnership
Brussels, Belgium, July 8th, 2021 – Ovizio Imaging Systems, a manufacturer of cell analyzers for advanced monitoring in the bioprocessing market, and BCC, an organization providing training, research services, and design of infrastructures for biopharmaceutical production, today announced that they will enter into a collaboration to broaden the range of monitoring tools for the bioprocessing industry.
The partnership will enable BCC to capitalize on Ovizio’s patented analyzers for label-free monitoring of bioproduction cell lines. In addition, Ovizio will leverage BCC’s expertise in the bioprocessing industry and process automation technologies to further optimize its monitoring algorithms and software integration with third-party instruments.
Dr. Emilie Viey, Ovizio’s CEO said, “This collaboration with BCC is an excellent opportunity for both of us to offer state-of-the-art training at the neighboring biopharmaceutical companies. We are confident that our joint efforts will ultimately facilitate the emergence of much-improved cGMP production plants with a better understanding of CQAs.
Dr. Emilie Viey, Ovizio’s CEO said, “This collaboration with BCC is an excellent opportunity for both of us to offer state-of-the-art training at the neighboring biopharmaceutical companies. We are confident that our joint efforts will ultimately facilitate the emergence of much-improved cGMP production plants with a better understanding of CQAs.”
About Ovizio Imaging Systems
Ovizio is an innovative company developing unique analyzers for advanced cell monitoring in bioprocessing applications. Its technology is based on proprietary, patented quantitative phase imaging and has the potential to track a broad range of phenotypic changes in a non-invasive, label-free manner throughout the complete cell culture process.
The analyzers capture images which provide information about physical cell parameters such as diameter, membrane regularity, granularity, and other features. These parameters are then combined through machine learning algorithms into a cell status signature, enabling monitoring of cell Critical Quality Attributes (CQAs) such as cell count, density, viability or % of aggregates, and can even track other phenotypical changes such as cellular response after viral transduction, immune cell activation, etc.
Applicable from R&D through to manufacturing processes and enabling significant cost reductions and quality improvement, Ovizio’s solutions are particularly convenient and robust for automated cell culture monitoring, analysis and quality control.
Follow Ovizio on LinkedIn
About the iLine F PRO
The iLine F PRO is a cGMP analyzer for on-line monitoring of cell status such as cell count and cell viability. Analysis relies on quantitative phase imaging – a type of label-free analysis – for acquisition of cellular parameters.
Data analysis is performed through machine learning algorithms with Ovizio’s proprietary software, OsOne. The software is automation friendly and can be connected to an infinite range of third-party instruments through the OPC-UA protocol. To enable a new level of automation, the iLine F PRO is equipped with a custom-designed, proprietary connection tubing enabling its closed-loop connection with most common bioreactors: benchtop stirred tanks and waves, as well as larger bioreactors.
The iLine F PRO has been tested and approved by many mid- to large-scale biopharmaceutical companies to monitor cell count and viability. Recently, the instrument has gained interest for its ability to track various phenotypes in real time and potentially monitor transduction-induced phenotype alterations for vaccine and viral vector production.
About BCC
The Biofactory Competence Centre (BCC) is a unique, organization based in Fribourg, Switzerland, which provides theoretical and practical training, services and research collaborations and designs infrastructures for biopharmaceutical production. The BCC is a state-of –the-art modular facility of over 480m2 including upstream suites for both mammalian and microbial cell culture, a downstream suite for separation processes, primary recovery, chromatographic and filtration skids, analytical laboratories, cell banking as well as lecture rooms and offices. The facilities are designed to simulate cGMP operating conditions, to provide clients with an understanding of the working conditions in pharmaceutical production environments. All training and services are offered in English, German and French.
Visit: www.bcc.ch/
Media contact:
Thomas Guyon
info@ovizio.com
CanCell Therapeutics partners with Ovizio to improve monitoring of CAR-T cell production
Share this
About the partnership
Brussels, Belgium, June 14, 2021 – Ovizio Imaging Systems, a manufacturer of cell analyzers for advanced monitoring in the bioprocessing market, and CanCell Therapeutics, an early-stage biopharmaceutical company focused on the development of CAR-T cell therapies, today announced that they will enter into a collaboration to further improve the monitoring of CAR-T cell-therapy manufacturing.
The partnership will enable CanCell Therapeutics to capitalize on Ovizio’s patented analyzers for label-free monitoring of CAR-T cell manufacturing. In addition, Ovizio will leverage CanCell Therapeutics’ expertise in CAR-T cell manufacturing to further optimize its CAR-T monitoring machine learning algorithms. CAR-T production involves T-cell purification and amplification, viral transduction to achieve CAR expression, then formulation, fill and finish to achieve a deliverable cell therapy product. During amplification and CAR transduction, cells undergo significant morphology changes reflecting their progressive transformation to the desired end product. This morphological change can be monitored using Ovizio’s unique and patented technology.
Dr. Emilie Viey, Ovizio’s CEO said, “This collaboration with CanCell Therapeutics is an excellent opportunity for us to contribute to their development of crucial CAR-T therapies. At the same time, we look forward to training our machine learning algorithms to better understand the morphological changes occurring in these cells. Both of these benefits will ultimately facilitate the production of better cell therapies.”
About Ovizio Imaging Systems
Ovizio is an innovative company developing unique analyzers for advanced cell monitoring in bioprocessing applications. Ovizio’s technology has the potential to track a broad range of phenotypic changes in a label-free manner. Ovizio’s analyzers enable cell density and cell viability assessment and have the potential to track many other phenotypical changes such as CAR transduction efficiency and lymphocyte activation.
The technology is based on proprietary, patented quantitative phase imaging to enable non-invasive, label-free imaging of cells in culture. The captured images are acquired through patented technology and provide information on cell parameters such as diameter, membrane regularity, granularity, and other features. These parameters are then combined through machine learning algorithms into a cell status signature, enabling monitoring of cell Critical Quality Attributes (CQAs) such as cell count, viability, % of aggregates etc. throughout the complete cell culture process. Applicable from R&D through to manufacturing processes and enabling significant cost reductions and quality improvement, Ovizio’s solution are particularly convenient and robust for automated cell culture monitoring, analysis and quality control. Follow Ovizio on LinkedIn
About the qMod
The qMod is a camera that enables label-free monitoring of cell status such as cell count and cell viability. It relies on quantitative phase imaging for acquisition of cellular parameters and machine learning algorithms for phenotypic monitoring. It can be attached to brightfield microscopes equipped with a c-mount port and is compatible with a wide range of magnifications. Images are acquired on the qMod camera and automatically transferred to a nearby computer through a USB connection. Data analysis is performed with Ovizio’s proprietary software, OsOne.
About CanCell Therapeutics
The immune system constitutes a real barrier against exogenous pathogens as well as endogenous danger signals such as abnormal cancer cells. Unfortunately, the immune system alone is not always strong enough. Manipulation of certain immune cells allows them to be specifically reprogrammed and redirected against a tumor to eliminate it, creating the innovative cellular drugs of tomorrow. Based on 20 years of experience in cell and gene therapy, Dr. Marina Deschamps and Dr. Christophe Ferrand have co-founded CanCell Therapeutics, a spin-off of the French Blood Center (EFS Bourgogne Franche-Comté, France). CanCell Therapeutics aims to develop, at clinical stage, a chimeric antigen receptor (CAR) T cell therapy targeting leukemia cells. In the long term, they aim to support other immunotherapy projects in the field towards clinical application.
Visit: deca-bfc.com/index.php/portfolio-items/cancell-therapeutic/
Media contact:
Thomas Guyon
info@ovizio.com
Optical Investigation of Individual Red Blood Cells for Determining Cell Count and Cellular Hemoglobin Concentration in a Microfluidic Channel
Siemens Healthcare demonstrate a blood analysis routine by observing red blood cells through light and digital holographic microscopy in a microfluidic channel. With this setup a determination of red blood cell (RBC) concentration, the mean corpuscular volume (MCV), and corpuscular hemoglobin concentration mean (CHCM) is feasible. Cell count variations in between measurements differed by 2.47% with a deviation of −0.26×106µL to the reference value obtained from the Siemens ADVIA 2120i. Measured MCV values varied by 2.25% and CHCM values by 3.78% compared to the reference ADVIA measurement. Their results suggest that the combination of optical analysis with microfluidics handling provides a promising new approach to red blood cell counts.
Cultivating Multidisciplinarity: Manufacturing and Sensing Challenges in Cultured Meat Production
United Nations estimates that by the year 2050, the population of nearly 10 billion people will have 70% higher food demands than the current food systems can provide for. This needs to be observed in the context of the on-going climate change and related negative effects of the traditional agriculture. Conventional livestock-based value chains contribute to the high greenhouse gas emissions. Meat cultivation via cellular agriculture holds great promise as a method for future food production. Theoretically, it is an ideal way of meat production, humane to the animals and sustainable for the environment, while keeping the same taste and nutritional values as traditional meat. However, in practice, there is still a number of challenges such as large-scale production, regulatory compliance and consumer acceptance. To address these challenges a multidisciplinary approach is necessary. In this optic, we present an overview of the sensor monitoring options for the most relevant parameters for cultured meat bioprocess. Various examples of the sensors to potentially apply in cultured meat production are provided, as well as the options for their integration into different types of bioreactors. Furthermore, we briefly present the current status of the cultured meat research and regulation, societal aspects and its commercialization.
