Background
The increasing threat of pandemics due to the appearance of new viral diseases and the increased spread of existing viruses globally urges the development of a flexible vaccine production platform. Virus like particles (VLPs) form a promising new vaccine concept and can be produced using the baculovirus expression vector system and insect cells. Online determination of the viable cell density and state of infection can help in determining the optimal parameters for the production process.
The iLine F microscope system continuously pumps suspension cells in culture through a measuring chamber in a closed-loop system and makes holographic images of the cells resulting in up to 70 parameters per individual cell. Thus, it can accurately determine cell concentration and cell shape. The next step is to study whether it can also measure the infection state of the cell.
Objective
Evaluate online 3D holographic microscopy (iLine F) to determine the fraction of infected cells.
Results
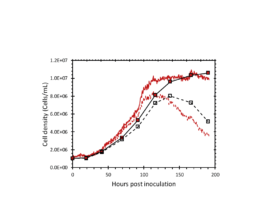
Figure 2: The total and viable cell density determined by the iLine F (red lines) and offline counts (square markers).
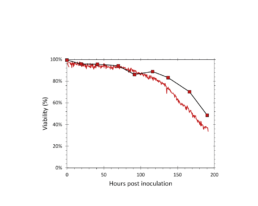
Figure 3: The viability determined by the iLine F (solid red) and trypan blue staining (square markers).
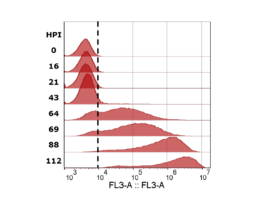
Figure 4: mCherry fluorescence measured with flow cytometer. Cells with values higher than the gate (dotted black line) were determined to be infected. Sample timepoints are given in hours post infection (HPI).
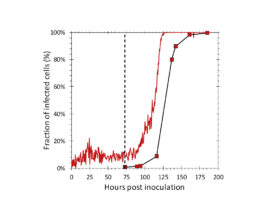
Figure 5: Fraction of infected cells determined by the iLine F (solid red) and the flow cytometer (square markers). AcBAC-PH-mCherry (MOI=0.1) was added to the reactor at 73 hours post inoculation (dotted black line).
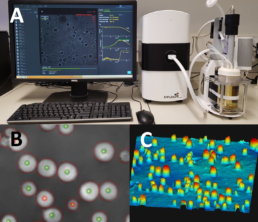
Figure 1. A: A single use bioreactor setup with the iLine F monitoring system. B: The cell segmentation and live dead determination performed by the microscope (green markers: live, red markers: dead). C: The 3D image created by the microscope.
Methods
• ExpiSf cells (Thermo Fisher) were cultured in ExpiSf chemically defined medium (Gibco)
• AcMNPVs: AcBAC-PH-HisGFP, AcBAC-PH-mCherry
• Applikon MiniBio reactors (400mL working volume, DO=30%, T=27⁰C)
• iLine F microscope system (OVIZIO)
• TC20 automated cell counter (Bio-Rad)
• Cells were infected at 3-4×10^6 cells/mL with a multiplicity of infection of 0.1 (TCID/cell)
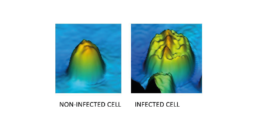
Figure 6: The 3D image made by the iLine F of a non-infected and infected Sf9 cell.
The iLine F showed a cell density and viability trend similar to the offline measurements. Figure 5 shows that the iLine F was able to detect baculoviral infection earlier than the offline flow cytometer method. Since mCherry was expressed behind the very late polyhedrin promoter (active at ~18 hours post infection) the flow cytometer method shown in Figure 4 and 5 only detected cells that were in the late stage of infection. Online holographic microscopy can capture changes directly as they occur on the cellular level, eliminating the need of offline sample preparation and reporter proteins such as mCherry. Detection is based on a combination of parameters generated by the microscope. A training of the algorithm was necessary (data not shown). With increasing amounts of data the algorithm can be further optimized and also used to re-analyze old data sets.
Conclusion
-
The iLine F microscope system is able to accurately estimate the fraction of baculoviral infected cells.
-
Cell infection can be detected in real time and earlier than offline methods.


Maarten Klaverdijk¹, Jort Altenburg¹, Dirk Martens¹, Gorben Pijlman², Jan van Hauwermeiren³, Jérémie Barbau³, Laurent Desmecht³, Damien Cabosart³
1. Bioprocess Engineering, Wageningen University and Research, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands,
2. Laboratory of Virology, Wageningen University and Research, Droevendaalsesteeg 1, 6708 PB Wageningen, The Netherlands,
3. Ovizio Imaging Systems, Rue du Bourdon 100/2 1180 Brussels, Belgium
