Advanced Therapies Week | January 25th – 28th, 2022
By bringing the industry together, Advanced Therapies Week offers you a unique opportunity to reflect upon the successes and failures from the last 12 months, enabling you to create actionable strategies and find new innovative technologies for the year ahead. With a focus on commercialization, the event provides the perfect blend of both suppliers and biotechs, who come together to share their technical expertise and ultimately help biotechs advance along their commercialization journey.Our CEO Emilie, together with Steve Wiltgen and Coleman Baker, will attend the event to showcase our iLine F PRO and see how it could benefit companies seeking to improve their bioprocess control and bioprocess automation strategies. You can find us at booth 804.
For more information, visit the event website
Ovizio Imaging Systems is entering into a commercial partnership with Merck to promote its iLine F PRO solution for the cell & gene therapy market
Brussels, Belgium, January 20, 2021 – Ovizio Imaging Systems, a life science company providing an innovative image-based cell analyzer for process automation and process control to the bioprocessing market, today enters into a commercial partnership with the Life Sciences Business Sector of Merck KGaA, Darmstadt, Germany, for its iLine F PRO solution.
Merck will ensure commercial promotion of the iLine F PRO solution, consisting of a cell analyzer and single-use disposable for companies working in the cell & gene therapy space in Europe and North America.
The partnership strategically aligns both companies’ decisive goals to simplify cell therapy manufacturing by combining the strength of the ekko™ Acoustic Cell Processing System, a multi-use platform for cell concentration and wash combined with aggregate processing, with Ovizio’s iLine F PRO cell analyzer, a Process Analytical Technology (PAT) providing real-time monitoring of cell expansion in a bioreactor.
Emilie Viey, PhD, Chief Executive Officer of Ovizio Imaging Systems: “For biologics, the link between the product and process is typically well defined and Cell Quality Attributes (CQAs) can be suitably characterized. In contrast, cell therapy programs – autologous cell therapy programs in particular – begin with highly complex and variable patient material, which necessitates more complex and diverse processes to generate a high-quality product. Offering automated and modular solutions to decrease variability and gain more understanding of the cell-based process is our driver. We couldn’t have wished for a better commercial partner to offer a solution to the major players in cell & gene therapy that covers delicate and key steps in their process.”
About Ovizio
Ovizio Imaging Systems develops and commercializes label-free cell analyzers designed for the bioprocessing market, with a focus on cell & gene therapy and vaccine production. We offer in-process analytical solutions that enable deep process understanding during development, and increased process control and consistency during manufacturing.
About the iLine F PRO
The iLine F PRO analyzer uses Ovizio’s proprietary label-free technology, a unique hologram-based method (patented Double Differential Digital Holographic Microscopy (D3HM)), for on-line, non-invasive classification and counting of suspension cells. Upon connection with a bioreactor, it continuously tracks objects in real-time at a single cell level with high accuracy, and enables automation of current manual processes. The iLine F PRO is cGMP compliant and is compatible with standard communication protocols (OPC UA) and its applications, such as Scada and DeltaV, enabling further process automation and control when used in conjunction with other devices.
The BioConnect is a single-use disposable that has been designed for closed loop fluidics. It allows the cells to circulate between the analyzer, where cells are imaged, and the bioreactor, where cells are grown. The risk of contamination is, therefore, drastically reduced and no biological material is wasted in the analysis process.
Learn more about the iLine F PRO Analyzer
The OsOne software relies on image-based processing algorithms to detect objects in suspension, and machine learning algorithms to classify these according to their optical and morphological signature. This ability removes the need for cell staining or labeling.
Learn more about our OsOne software
A few links for more information
MSC Profiling in Response to Priming
ASTEM and OVIZIO successfully implemented
a label-free method to monitor human bone marrow MSC morphological profile
Challenge
Mesenchymal stem cells (MSCs) are widely studied as a potential therapy for immune-mediated diseases (osteoarthritis, diabetes, multiple sclerosis, and Alzheimer’s disease). MSCs are known to modulate immune cells such as T cells, B cells, macrophages, microglia, and dendritic cells.
MSCs can be used in an allogeneic, off-the-shelf manner, and have been studied in over 600 clinical trials to date.
However, the manufacture of MSCs with consistent and predictable quality has proven difficult. There is an urgent need to develop imaging systems that are robust and provide real-time monitoring of attributes that are critical to the quality of the stem cells. It is of particular importance that such systems have the ability to not only monitor changes in stem cell phenotype but also their function. Moreover, such a system must also be able to account for donor-to-donor variability.
Solution
Ovizio’s qMod camera captures quantitative phase images from which 30 features related to stem cell phenotype can be extracted (aspect ratio, diameter, elongatedeness, etc). It does so in a label-free manner, easing the burden on quality
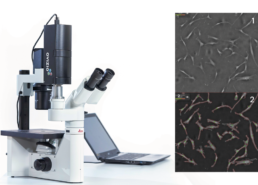
Phase image of MSC culture¹ with mask overlay²
The morphological features can be extracted from phase images and machine learning algorithms can be used to generate phenotypic signatures such as cell count, viability, priming status and immunosuppressive capacity.
Recently, software tools such as t-SNE have been developed to enable easy 2D visualization of complex data, facilitating exploratory research and potential identification of unknown populations.
Experimental Set-Up
We succeeded in establishing the optimal MSC seeding density and priming conditions to capture morphological changes in MSCs isolated from three donors.
We developed a preliminary algorithm to identify multiple MSC morphological features (cell aspect ratio, cell area, elongatedness, optical height) to predict the effects of IFN-γ and TNF-α priming. These observations show a
strong dependence on cell density, irrespective of donor.
Results
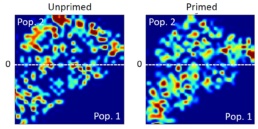
Applying the t-SNE algorithm on the data revealed subpopulation differences for all donors. When primed, there is an increase in MSC subpopulation 1 that corresponds to larger, more elongated, and flatter cells with a higher aspect ratio.
t-SNE algorithm testing on unprimed
and primed MSCs
The distribution of the four cell features in Donor 1 is illustrated above. Even though each donor has the same qualitative differences between Pop. 1 and 2, the feature distribution is donor-dependent.
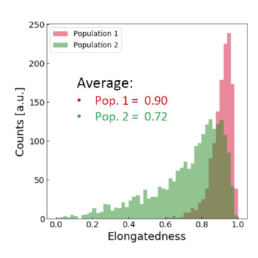
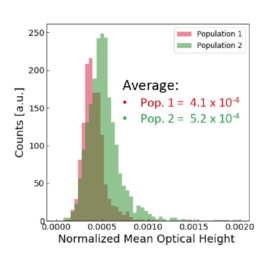
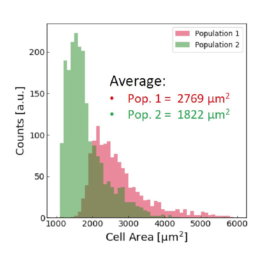
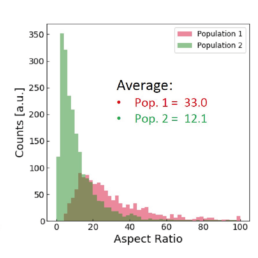
Using all available data, we built a supervised machine learning model (random forest classifier with shallow trees) using the computed cell features as input for the model. The preliminary supervised machine learning model concluded that the effect of priming on MSCs is not dependent on the donor. Rather our study showed:
- an increase in the cell aspect ratio (length/width)
- an increase in the cell area
- an increase in the elongatedness
- a decrease in the optical height
Supervised machine learning model testing on unprimed and primed MSCs
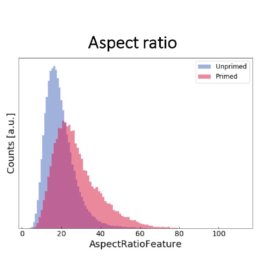
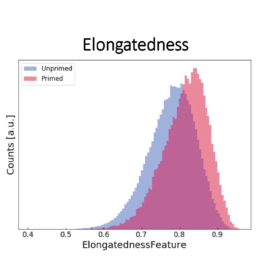
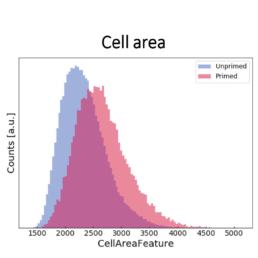
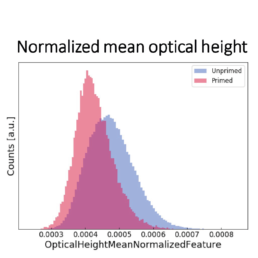
The image-based prediction model showed the distribution of the four cell features in unprimed (blue) and primed MSC populations (pink).
Conclusion
We developed a machine learning algorithm to predict the effect of priming on changes in MSC morphology. We show that primed MSCs are larger, more elongated, and have a flatter and higher aspect ratio.
These results will be further validated in a comprehensive study using MSCs isolated from 12 additional donors. This will include a correlation between MSC function and morphological changes in response to various priming events. Ultimately, this study seeks to position the qMod imaging system for monitoring the quality of MSCs in the manufacturing workflow.

Contact: Pascale Charbonnel, pascale.charbonnel@ovizio.com
Catherine Chong, chongyf@imcb.a-star.edu.sg
Steve Oh, Simon Cool, Ying Zhang, Yin Lu, Tongming Liu from ASTARSG
Damien Cabosart, Pascale Charbonnel, Emilie Viey from Ovizio
Biofactory Competence Centre partners with Ovizio to improve PAT manufacturing
Share this
About the partnership
Brussels, Belgium, July 8th, 2021 – Ovizio Imaging Systems, a manufacturer of cell analyzers for advanced monitoring in the bioprocessing market, and BCC, an organization providing training, research services, and design of infrastructures for biopharmaceutical production, today announced that they will enter into a collaboration to broaden the range of monitoring tools for the bioprocessing industry.
The partnership will enable BCC to capitalize on Ovizio’s patented analyzers for label-free monitoring of bioproduction cell lines. In addition, Ovizio will leverage BCC’s expertise in the bioprocessing industry and process automation technologies to further optimize its monitoring algorithms and software integration with third-party instruments.
Dr. Emilie Viey, Ovizio’s CEO said, “This collaboration with BCC is an excellent opportunity for both of us to offer state-of-the-art training at the neighboring biopharmaceutical companies. We are confident that our joint efforts will ultimately facilitate the emergence of much-improved cGMP production plants with a better understanding of CQAs.
Dr. Emilie Viey, Ovizio’s CEO said, “This collaboration with BCC is an excellent opportunity for both of us to offer state-of-the-art training at the neighboring biopharmaceutical companies. We are confident that our joint efforts will ultimately facilitate the emergence of much-improved cGMP production plants with a better understanding of CQAs.”
About Ovizio Imaging Systems
Ovizio is an innovative company developing unique analyzers for advanced cell monitoring in bioprocessing applications. Its technology is based on proprietary, patented quantitative phase imaging and has the potential to track a broad range of phenotypic changes in a non-invasive, label-free manner throughout the complete cell culture process.
The analyzers capture images which provide information about physical cell parameters such as diameter, membrane regularity, granularity, and other features. These parameters are then combined through machine learning algorithms into a cell status signature, enabling monitoring of cell Critical Quality Attributes (CQAs) such as cell count, density, viability or % of aggregates, and can even track other phenotypical changes such as cellular response after viral transduction, immune cell activation, etc.
Applicable from R&D through to manufacturing processes and enabling significant cost reductions and quality improvement, Ovizio’s solutions are particularly convenient and robust for automated cell culture monitoring, analysis and quality control.
Follow Ovizio on LinkedIn
About the iLine F PRO
The iLine F PRO is a cGMP analyzer for on-line monitoring of cell status such as cell count and cell viability. Analysis relies on quantitative phase imaging – a type of label-free analysis – for acquisition of cellular parameters.
Data analysis is performed through machine learning algorithms with Ovizio’s proprietary software, OsOne. The software is automation friendly and can be connected to an infinite range of third-party instruments through the OPC-UA protocol. To enable a new level of automation, the iLine F PRO is equipped with a custom-designed, proprietary connection tubing enabling its closed-loop connection with most common bioreactors: benchtop stirred tanks and waves, as well as larger bioreactors.
The iLine F PRO has been tested and approved by many mid- to large-scale biopharmaceutical companies to monitor cell count and viability. Recently, the instrument has gained interest for its ability to track various phenotypes in real time and potentially monitor transduction-induced phenotype alterations for vaccine and viral vector production.
About BCC
The Biofactory Competence Centre (BCC) is a unique, organization based in Fribourg, Switzerland, which provides theoretical and practical training, services and research collaborations and designs infrastructures for biopharmaceutical production. The BCC is a state-of –the-art modular facility of over 480m2 including upstream suites for both mammalian and microbial cell culture, a downstream suite for separation processes, primary recovery, chromatographic and filtration skids, analytical laboratories, cell banking as well as lecture rooms and offices. The facilities are designed to simulate cGMP operating conditions, to provide clients with an understanding of the working conditions in pharmaceutical production environments. All training and services are offered in English, German and French.
Visit: www.bcc.ch/
Media contact:
Thomas Guyon
info@ovizio.com
CanCell Therapeutics partners with Ovizio to improve monitoring of CAR-T cell production
Share this
About the partnership
Brussels, Belgium, June 14, 2021 – Ovizio Imaging Systems, a manufacturer of cell analyzers for advanced monitoring in the bioprocessing market, and CanCell Therapeutics, an early-stage biopharmaceutical company focused on the development of CAR-T cell therapies, today announced that they will enter into a collaboration to further improve the monitoring of CAR-T cell-therapy manufacturing.
The partnership will enable CanCell Therapeutics to capitalize on Ovizio’s patented analyzers for label-free monitoring of CAR-T cell manufacturing. In addition, Ovizio will leverage CanCell Therapeutics’ expertise in CAR-T cell manufacturing to further optimize its CAR-T monitoring machine learning algorithms. CAR-T production involves T-cell purification and amplification, viral transduction to achieve CAR expression, then formulation, fill and finish to achieve a deliverable cell therapy product. During amplification and CAR transduction, cells undergo significant morphology changes reflecting their progressive transformation to the desired end product. This morphological change can be monitored using Ovizio’s unique and patented technology.
Dr. Emilie Viey, Ovizio’s CEO said, “This collaboration with CanCell Therapeutics is an excellent opportunity for us to contribute to their development of crucial CAR-T therapies. At the same time, we look forward to training our machine learning algorithms to better understand the morphological changes occurring in these cells. Both of these benefits will ultimately facilitate the production of better cell therapies.”
About Ovizio Imaging Systems
Ovizio is an innovative company developing unique analyzers for advanced cell monitoring in bioprocessing applications. Ovizio’s technology has the potential to track a broad range of phenotypic changes in a label-free manner. Ovizio’s analyzers enable cell density and cell viability assessment and have the potential to track many other phenotypical changes such as CAR transduction efficiency and lymphocyte activation.
The technology is based on proprietary, patented quantitative phase imaging to enable non-invasive, label-free imaging of cells in culture. The captured images are acquired through patented technology and provide information on cell parameters such as diameter, membrane regularity, granularity, and other features. These parameters are then combined through machine learning algorithms into a cell status signature, enabling monitoring of cell Critical Quality Attributes (CQAs) such as cell count, viability, % of aggregates etc. throughout the complete cell culture process. Applicable from R&D through to manufacturing processes and enabling significant cost reductions and quality improvement, Ovizio’s solution are particularly convenient and robust for automated cell culture monitoring, analysis and quality control. Follow Ovizio on LinkedIn
About the qMod
The qMod is a camera that enables label-free monitoring of cell status such as cell count and cell viability. It relies on quantitative phase imaging for acquisition of cellular parameters and machine learning algorithms for phenotypic monitoring. It can be attached to brightfield microscopes equipped with a c-mount port and is compatible with a wide range of magnifications. Images are acquired on the qMod camera and automatically transferred to a nearby computer through a USB connection. Data analysis is performed with Ovizio’s proprietary software, OsOne.
About CanCell Therapeutics
The immune system constitutes a real barrier against exogenous pathogens as well as endogenous danger signals such as abnormal cancer cells. Unfortunately, the immune system alone is not always strong enough. Manipulation of certain immune cells allows them to be specifically reprogrammed and redirected against a tumor to eliminate it, creating the innovative cellular drugs of tomorrow. Based on 20 years of experience in cell and gene therapy, Dr. Marina Deschamps and Dr. Christophe Ferrand have co-founded CanCell Therapeutics, a spin-off of the French Blood Center (EFS Bourgogne Franche-Comté, France). CanCell Therapeutics aims to develop, at clinical stage, a chimeric antigen receptor (CAR) T cell therapy targeting leukemia cells. In the long term, they aim to support other immunotherapy projects in the field towards clinical application.
Visit: deca-bfc.com/index.php/portfolio-items/cancell-therapeutic/
Media contact:
Thomas Guyon
info@ovizio.com
Online monitoring of growth and cell shape of CHO cells in stirred bioreactors
Mischa Stalder, Rüdiger Maschke, Regine Eible and Dieter Eible, ZHAW, presented their research results with a poster during BioTech 2017 at Zürich.
Automated Monitoring of CAR-T Cells in a Rocking Motion Bioreactor
Jérémie Barbau, Laurent Desmecht, Serge Jooris.
Ovizio Imaging Systems SA/NV, Brussels, Belgium
Introduction
Chimeric Antigen Receptor (CAR) T-cell therapies are showing high response rates in patients worldwide, resulting in two approved products by the US Food and Drug Administration in 2017. T-cells from a patient are removed from the blood and engineered to express the CAR to reprogram the T-cells to target patients tumoral cells. (1)
In these autologous therapies, the challenges lay in the inherent variability in starting materials and the goal of maximizing product consistency while producing a safe, pure and potent product. Cell counting is one of the most fundamental metrics of it. With the development of Cell Therapy Products (CTPs), there is an increased need for robust and validated measurements for cell characterization to enable manufacturing control and absafe/high-quality product suitable to be released to the patients. (2)
In CAR-T cell manufacturing, each handling or addition of reagents to the cell preparation generates a risk for error and for contamination that can possibly lead to the loss of a production run. A reliable solution consists of removal of open handling and implementing closed culture systems, where the cell manufacturing takes place in bags with closed tubing pathways and connections, maintaining a sterile environment. Ovizio’s patented technology, Double Differential Digital Holographic Microscopy (D3HM), is a quantitative imaging technique that allows cell monitoring in a continuous, automated and label-free set-up. No need for sampling (eliminating the risk of contamination), staining, and waiting for results generated by an off-line counter or analyzer; therefore, results are available in nearly real-time and continuous spanning the length of the culture. The platform generates a holographic fingerprint based on 70 parameters for every cell that is imaged, and feeds this data to a machine learning platform that can be trained to identify cells and discriminate between cell types. Fast and accurate, the algorithms automatically discriminate living from dead cells, count and give access to in-depth quality attributes and dynamic properties of your samples, and may also provide additional information on a single cell level.
In this application note, the use of the iLine F, manufactured by Ovizio Imaging Systems, for the monitoring of T-cells as they grow in a rocking motion bioreactor is described. The iLine F delivers reliable measurements of viability, cell density, diameter evolution and specific cellular critical quality attributes, allowing for real time in-process controls. Typically, the iLine F (i) provides a continuous monitoring of T cells culture in wave bags, (ii) counts and discriminates the viability of the T cells, (iii) gives strongly comparable Total Cell Density (TCD) and Viable Cell Density (VCD) with an off-line reference counting method (R2 <0.95) and, (iv) tracks small phenotype changes allowing for a T lymphocytes classification (subsets, differentiation states (still under evaluation)).
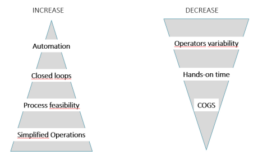
Figure 1. The needs and expectations associated with manufacturing of Cell Therapy Products.
Ovizio’s technology
Digital Holographic Microscopy is the scattered light beam from an illuminated object that interferes with a reference beam on a CCD camera allowing for a 3D numerical reconstruction of that object. Double Differential Digital Holography (D3HM) (Figure 2) is an evolution of this base technology that enables the use of a partial coherent light source (low power, non-invasive LED light, e.g.) resulting in improved image quality as well as an important size reduction of the instruments. A patented method is used to discriminate live from dead cells: a living cell containing cytoplasm is spherical and will create an out of focus light cone, whereas a dead cell losing membrane integrity and cytoplasm will diffuse light (Figure 2). One of the most important features of holographic microscopy is the capability of refocusing out of focus objects post acquisition. This makes the platform extremely suited of tracking objects in suspension.
The iLine F microscope acquires both intensity and quantitative phase contrast information of a microscopic sample allowing the automated extraction of 70 parameters for each object captured- the object being a cell (dead or alive).
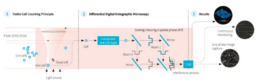
Figure 2. Ovizio’s Double Differential Holographic Imaging Technology. Live or dead cells are the objects (1) that pass
through the light scattering and interference process (2) resulting in the 3D reconstruction and quantitative phase data (3).
The iLine F can perform continuous suspension cell monitoring in rocking motion (wave) type bioreactors via an adapted setup (Figure 5). The disposable BioConnect sampling probe in combination with a reusable pump is a closed loop that pumps cells out of the wave bag through a flow cell that inserts into the iLine F microscope, holographic data is then acquired, and cells flow back into the wave bag. The holograms are analyzed by OsOne, the monitoring software part of the iLine F microscope and a holographic fingerprint is generated for every cell found within the hologram. The fingerprint is then used by a machine learning platform trained to identify, count and analyze the sample. Viability, transfection kinetics and other cellular parameters can be detected by the platform.

Figure 3. Machine learning principle.
The system can be trained to discriminate and count different subpopulations by observing pure populations (100% population “A”) and then observing known mixtures of that same subpopulation in incremental steps (90%, 80%, 70%, … 10%, e.g.). The system can either automatically, or assisted by supervised learning methods, define which parameters are best to discriminate the sub populations.
Materials
- iLine F D3HM device
- Single use BioConnect monitoring probe
- Single use adapter for disposable bags
- Reusable pump engine
- OsOne Software version 5.12
- GE Healthcare Xuri Cell Expansion System W25
- GE Healthcare Cellbag 2L DOOPT II, pHOPT and Perfusion (reference 29-1054-98)
- An offline reference counting method making use of fluorescent dyes
Methods
The BioConnect, Ovizio’s disposable sampling probe for bioreactor monitoring is directly connected to a disposable cell culture bag. Two silicone tubes that terminate with C-flex and male Luer locks are used to connect to the wave bag, either by welding or under sterile conditions (within a LAF hood). The BioConnect was sterilized in an autoclave for 20 minutes at 120°C and connected aseptically to the cell
culture bag using welding.
The inlet tubing of the BioConnect is connected to the harvest line in order to take advantage of the dip tubing in the cell culture bag while the outlet can be connected to the sampling port. Sampling was still possible by adding a single use (sterilized by autoclave), T-shaped adapter between the sample port and the BioConnect.
Figure 4. Modified BioConnect.
Figure 5. Complete wave bioreactor setup.
Once the assembly finalized, OsOne software was launched and a wizard designed to guide users through the selections of the proper cell type, and the number of data points to be acquired during the run, was completed.
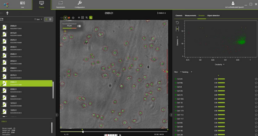
Figure 6. OsOne software.
OsOne is an all-in-one software that:
- controls the iLine F microscope
- acquires holographic fingerprints of each individual cell within the wave bag
- computes the results (data analysis)
Cell density, viability and the growth curves (viable and total cell density) are displayed on the screen for easy viewing while operating. The bioreactor was inoculated with 1×106 viable T cells/ml. For this experiment, the iLine F has been set to acquire holograms every 2 minutes and computes a data point every hour. At low cell densities the system automatically doubles the number of images captured to increase statistical relevance. For every single cell imaged by the platform, 70 parameters are recorded and available for additional post processing if required.
RESULTS
OsOne plots results graphically on the screen in nearly real-time (Figure 6). During the complete run, images of the cells are acquired every two minutes. Data can be exported in multiple formats and archived for future analyses.
During the experiment data was acquired for up to 4 days continuously by the iLine F microscope and was compared to results generated by the off-line reference instrument. On average, two manual samples were acquired for off-line analyses per day.

Figure 7. Total Cell Density and Viable Cell Density compared to the off-line reference method.
Figure 7 illustrates Total Cell Density (TCD) and the Viable Cell Density (VCD) compared to the reference method used. After plotting the data points, the correlation coefficient (R2 ) was calculated and resulted in a value of 0.96. This measurement represents the actual deviation between the compared methods with respect to a linear correlation assumption, that is, if cell densities grow by a factor of two, then both methods are expected to report a factor of two. A perfect correlation results in an R2 value of 1, though, it does not indicate to whether
the absolute measurements are the same.
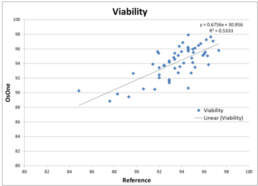
Figure 8. Viability compared to the off-line reference method.
Figure 8 illustrates Viability as provided by the iLine F compared to the reference method used. After plotting the data points, the correlation coefficient (R2 ) was calculated and resulted in a value of 0.53. This value is lower compared to the value obtained when comparing TCD and VCD with the reference method. It can be explained by the fact that viability stays around 95% during the course of the culture. Indeed very few data points can be found below 90%. It results in the values being part of a cloud of points instead of being distributed along a line. As a consequence, fitting a regression line is much more complicated hence the low value for the correlation coefficient. However, the discrepancies between the values generated by the iLine F and the reference method are minor: mostly within 5% of variability.

Figure 9. Total Cell Density and Viable Cell Density compared to the off-line reference method within a 95% confidence
interval.
A 95% confidence interval has been fitted on the Total Cell Density and the Viable Cell Density, showing that most of the values are within this confidence interval.

Figure 10. Donor-related variation shows that there is no influence from the donor on the quality of the measurement.
Donor-related variations have been investigated. Figure 10 gives an overview of 3 different donors. Differences in the cell growth pattern can be seen while the correlation between the data provided by the iLine F and the reference method remain very good for each donor. It shows that the system is able to produce reliable data, independently of the donor.
It has to be noted that at the start of the run, there are less cells and thus more empty images, therefore, we observed some variability in the results when compared to the first three off-line data points. After 24 hours the correlations improved as the density increased with culture growth. Improvements have been made in the OsOne software to acquire more images at low densities to mitigate this limitation.
CONCLUSION
This study illustrates the robustness and reliability of Ovizio’s label-free approach for T-cell based expansion in process development or manufacturing environment. We have addressed the need to understand the biological basis for cell counting and characterization, especially when a subpopulation of cells is hypothesized to correlate with a clinical outcome.
The iLine F, in-line technology combined with the machine learning based analysis software OsOne, delivers results that are nearly equivalent to traditional off-line methods. Moreover, it provides additional information on a single cell level for continuous monitoring of CAR-T cell cultures. As the system is automated, there is also no need for an operator to sample the cell culture bag, thus eliminating operator-related variability and reducing operational costs.
The iLine F and adapted BioConnect disposables can be used as a Process Analytical Tool in a closed manufacturing environment with improved product characterization maintaining sterility throughout the cell expansion process. The instrument can be connected to the manufacturing control environment via OPC and is CFR 21 part 11 compliant. Each run is archived, and data can be accessed for analyses or regulatory purposes.
REFERENCES
- Towards a commercial process for the manufacture of genetically modified T cells for therapy, A D Kaiser, M Assenmacher, B Schröder, M Meyer, R Orentas, U Bethke & B Dropulic, Cancer Gene Therapy volume 22, pages 72–78 (2015)
- Manufacturing Cell Therapies: The Paradigm Shift in Health Care of This Century, Rachel Haddock, MS, GlaxoSmithKline; Sheng Lin-Gibson, PhD, National Institute of Standards and Technology; Nadya Lumelsky, PhD, National Institute of Dental and Craniofacial Research, National Institutes of Health; Richard McFarland, PhD, MD, Advanced Regenerative Manufacturing Institute; Krishnendu Roy, PhD, Georgia Institute of Technology; Krishanu Saha, PhD, University of Wisconsin– Madison; Jiwen Zhang, PhD, GE Healthcare; Claudia Zylberberg, PhD, Akron Biotech, June 23, 2017
Real-time and label-free automated cell monitoring: Shed light on your cells
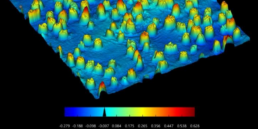
Any lab faces bottleneck challenges when scaling up activities.
When scaling up your lab activities, bottlenecks can occur in multiple phases of handling cells in culture vessels:
- manual sampling
- sample preparation
- staining
- feed to offline instrument
- readout by trained operator
- disposal of sample

On-line and dye-free automated cell monitoring: bring automation to cell expansion
Ovizio’s value proposition:
- A closed loop cell monitoring system decreases biosafety risks.
- Real-time cell culture analysis increases quality control and improves proces controls.
- Automation and dye-free analysis of cell culture decreases development costs and operating costs.
The connection to our cell culture analyzer is bioreactor agnostic.
Our system can connect with many types of bioreactors:
- small scale bioreactors
- single use bioreactors
- rocking motion bioreactors
- benchtop bioreactors
- stainless steel bioreactors
Different bioreactor connection types are supported:
- c-flex or pvc welding
- luer lock connection
- novaseptum sterile connection

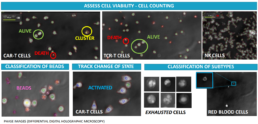
3D image based automated cell monitoring provides immune cell fingerprints.
Our cell culture analysis system uses differential digital holographic microscopy for:
- assessing cell viability
- cell counting
- classification of beads
- tracking changes of state
- classification of subtypes
Automated, closed-loop, in-line monitoring of CAR-T cells in a production process
Double Differential Digital Holographic Microscopy (D3HM)
Cell-based technology is a fundamental pillar of modern biotechnology. Cell counting is one of the most fundamental metrics of it. With the development of Cell Therapy Products (CTPs), there is an increased need for robust and validated measurements for cell characterization to enable manufacturing control and a safe/high-quality product released to the patients .
Our company has developed an in-line, automated microscope to monitor in real-time the suspension culture in a bioreactor. Its versatility makes it compatible with off-the-shelf stirred tanks, wave bags and others. The cell characterization and quantification are based on OVIZIO’s patented technology: Double Differential Digital Holographic Microscopy (D3HM). The microscope generates a holographic fingerprint based on 70 parameters for every cell that is imaged and feeds to a machine learning platform. Fast and accurate, the algorithms automatically discriminate living from dead cells, count and give access to in-depth quality attributes and dynamic properties of your samples, and may also provide additional information on a single cell level.
In this study we show that our in-line microscope delivers a continuous monitoring of T cells culture in wave bags, counts and discriminate the viability of the T cells, gives strongly comparable Total Cell Density (TCD) and Viable Cell Density (VCD) with an off-line reference counting method (0,93> R2 >0.99) and, tracks small phenotype changes allowing for a T lymphocytes classification (subsets, differentiation states (still under evaluation)).
This study illustrates the robustness and reliability of OVIZIO’s label-free approach for T-cell based expansion in process development or
manufacturing environment. We have addressed the need to understand the biological basis for cell counting, especially when a subpopulation of cells is hypothesized to correlate with a clinical outcome.